Mechanism of Action
When combined with an antiviral, CYTOGAM® (Cytomegalovirus Immune Globulin Intravenous [Human]) (CMV-IGIV) provides prophylaxis against CMV infection or disease in immunocompromised solid organ transplant patients.1
After Transplant, CMV-IGIV May Augment a Standard Antiviral to Improve Control of CMV Through Its Mechanism of Action2-4
CYTOGAM is an intravenous, purified hyperimmune globulin enriched in immunoglobulin G (IgG) antibodies against CMV.5
As a hyperimmune globulin, CYTOGAM may offer a complementary approach to antiviral therapy by acting outside the cell.3,6
The mechanism of action (MOA) for hyperimmune globulins involves multiple simultaneous actions3,6,7
Neutralization and virolysis: Preventing CMV from infecting healthy cells and the complement-dependent virolysis of free CMV particles3,8-10
Opsonization: Marking CMV for destruction and removal via phagocytosis3
Cytolysis: Facilitating antibody-dependent cellular cytotoxicity (ADCC) and membrane attack complex (MAC)-dependent cytolysis of CMV-infected cells3,11-13
Immune modulation: Inhibiting proinflammatory cells and reducing cytokine secretion6,14
Neutralization and Virolysis3,8,9
CYTOGAM neutralizes free viral particles and interferes with their entry into cells as well as causes the destruction of the free-floating virus particles.
The antibody binding to the virus facilitates subsequent binding and activity of antibody-dependent virolysis.

Opsonization3
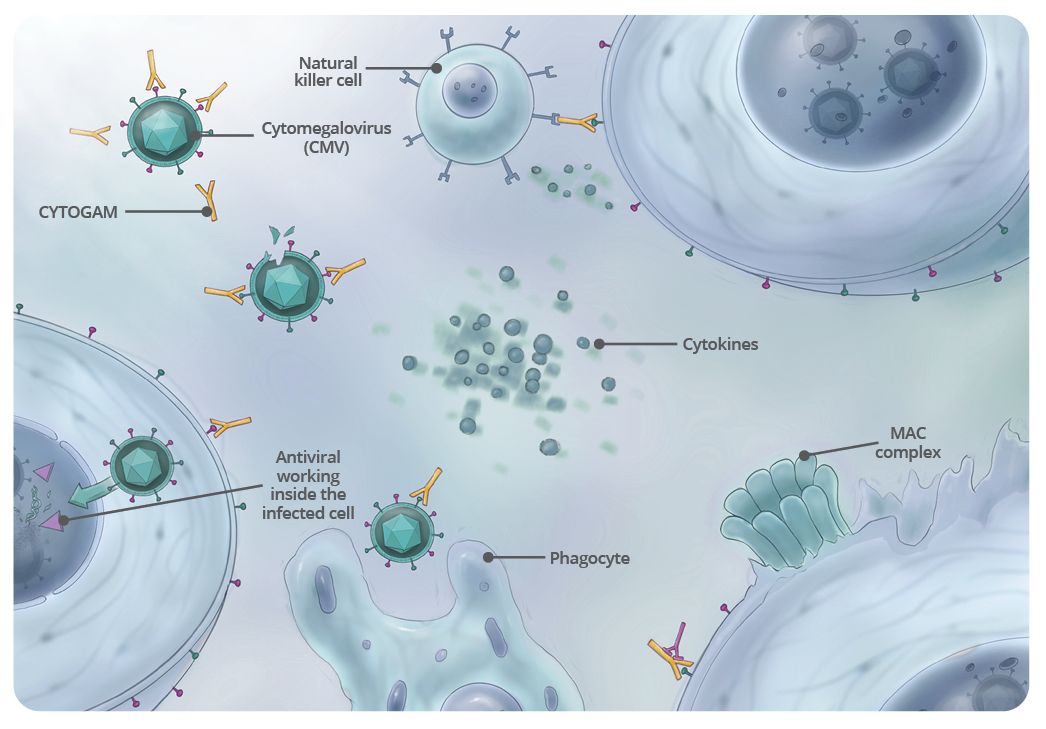
Binding of anti-CMV antibody to CMV is a form of opsonization, which then promotes phagocytosis of the virus. This type of opsonization involves the binding of CYTOGAM molecules to epitopes on the surface of CMV viral particles. After this binding, phagocytes are attracted to CMV.
A phagocyte approaches the opsonized CMV, and the Fc region of CYTOGAM binds to one of the Fc receptors on the surface of the phagocyte.
Cytolysis3,9,11-13
IgGs play a role in the destruction of cells infected with CMV.
Cytolysis (ADCC)
Antibody-dependent cellular cytotoxicity (ADCC) occurs when the antibody binds to an infected cell, then engages the Fc receptor on an immune effector cell (such as a natural killer cell). This immunologic bridge triggers the release of cytotoxic granules that destroy the infected cell.

Cytolysis (MAC-dependent)
Antibodies bind to viral proteins on the surface of infected cells, then are cross-linked by antibody-dependent virolysis. This action causes an enzymatic cascade, culminating in membrane attack complex (MAC)-dependent cell lysis.

Immune Modulation6,14
CYTOGAM, as an IgG, exerts enhancing and suppressive immunomodulatory functions that may help to control some of the direct and indirect effects of post-transplant CMV infection.
- The immunomodulatory properties of CYTOGAM have the potential to inhibit proinflammatory cells, which:
- Reduces levels of inflammatory mediators by reducing cytokine production and secretion, and therefore
- Potentially lowers the risk of organ rejection
In vitro data and a study in transplant patients have shown that the application of CMV-IGIV decreases production of the cytokines interleukin (IL)-2, interferon (IFN)‐γ, IL-6, and IL-10.
Learn more about the MOA for CYTOGAM in a video that highlights how CYTOGAM works in the body and helps prevent CMV.
Watch this Video
References: 1. Pelletier JPR, Faisal Mukhtar F. Passive Monoclonal and Polyclonal Antibody Therapies. In: Immunologic Concepts in Transfusion Medicine. Elsevier Health. 2020;16:251-358. 2. Majewska A, Młynarczyk-Bonikowska B, Malejczyk M, Majewski S, Młynarczyk G. Possibilities of prevention and treatment of human cytomegalovirus infections including new drugs and compounds with potential application. Advancements Microbiol. 2019:58(3):291-299. 3. Grossi PA, Mohacsi P, Szabolcs Z, Potena L. Cytomegalovirus immunoglobulin after thoracic transplantation: an overview. Transplantation. 2016;100:S1-S4. 4. Valantine HA, Luikart H, Doyle R, et al. Impact of cytomegalovirus hyperimmune globulin on outcome after cardiothoracic transplantation: a comparative study of combined prophylaxis with CMV hyperimmune globulin versus ganciclovir alone. Transplantation. 2001;72(10):1647-1652. 5. CYTOGAM [package insert]. Kamada Inc.; September 2022. 6. Carbone J. The immunology of posttransplant CMV infection: potential effect of CMV immunoglobulins on distinct components of the immune response to CMV. Transplantation. 2016;100:S11-S18. 7. Deml L, Huber CM, Barabas S, Spindler T, Cozzi E, Grossi P. Stimulatory effect of CMV immunoglobulin on innate immunity and on the immunogenicity of CMV antigens. Transpl Dir. 2021;7:e781. doi:10.1097/TXD.0000000000001236 8. Cui X, Lee R, Adler SP, McVoy MA. Antibody inhibition of human cytomegalovirus spread in epithelial cell cultures. J Virolog Methods. 2013;192(1-2):44-50. 9. Bonaros NE, Kocher A, Dunkler D, et al. Comparison of combined prophylaxis of cytomegalovirus hyperimmune globulin plus ganciclovir versus cytomegalovirus hyperimmune globulin alone in high-risk heart transplant recipients. Transplantation. 2004;77(6):890-897. doi:10.1097/01.tp.0000119722.37337.dc 10. Mellors J, Carroll M. Direct enhancement of viral neutralising antibody potency by the complement system: a largely forgotten phenomenon. Cell Mol Life Sci. 2024;81(1):22. 11. Nelson CS, Baraniak I, Lilleri D, Reeves MB, Griffiths PD, Permar SR. Immune correlates of protection against human cytomegalovirus acquisition, replication, and disease. J Infect Dis. 2020;221(Suppl 1):s45-s59. 12. Chandler TL, Yang A, Otero CE, Permar SR, Caddy SL. Protective mechanisms of nonneutralizing antiviral antibodies. PLoS Pathog. 2023;19(10):e1011670. 13. Semmes EC, Miller IG, Rodgers N, et al. ADCC-activating antibodies correlate with decreased risk of congenital human cytomegalovirus transmission. JCI Insight. 2023;8(13):e167768. 14. Kornberg A, Witt U, Kornberg J, et al. Prophylactic anti-cytomegalovirus hyperimmunoglobulin in critically ill liver transplant patients: impact on early immunology and survival. J Clin Med. 2020;9(3):656.